CPD aligned • Evenings • UK‑based
PharmaLink Accelerator Programme
12 weeks to navigate Clinical, Regulatory, Pharmacovigilance, Medical Affairs and HEOR. Build confidence through a cross‑functional simulation, weekly evening classes and 1 to 1 expert feedback. Finish with a capstone and a clear job strategy.
projects
feedback
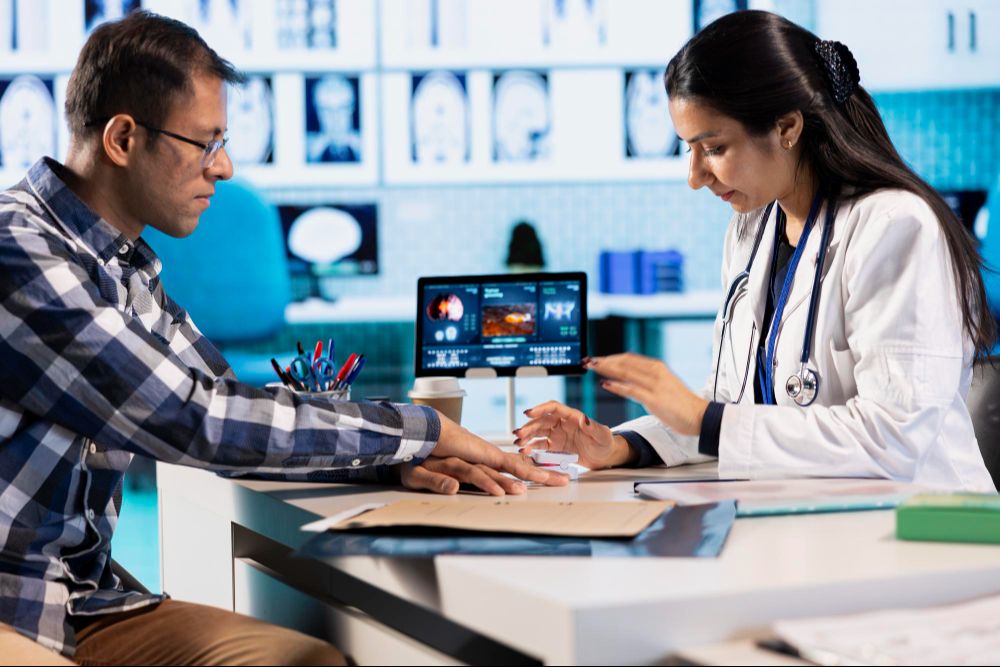
CPD aligned
Weekly evening classes
Industry-standard projects
Alumni community
Next cohorts
Evening classes run 6 to 8 pm. Intake is limited for quality feedback.
What you will produce
Your cross‑functional portfolio demonstrates breadth and readiness for entry‑level roles.

Clinical mini‑protocol excerpt
Objectives, endpoints and concise visit schedule rationale.

Regulatory writing sample
Module 2 summary or CSR results section with QC traceability.

Safety and MA artefacts
SUSAR flow note and a scientific communication one‑pager.
- Inspection readiness checklist with CAPA example
- HEOR value summary for the mock asset
- Personal portfolio and interview prep notes
Syllabus
Week 1: Pharma Industry Immersion & Drug Development Orientation
Week 2: Regulatory Systems & Compliance
Week 3: Cross‑Functional Simulation Kick‑Off
Week 4: Clinical Development Deep Dive
Week 5: Pharmacovigilance & Drug Safety
Week 6: Regulatory Writing & Submissions
Week 7: Medical Affairs & Scientific Communication
Week 8: Health Economics & Outcomes Research (HEOR)
Week 9: Inspections, Audits & Quality Management
Week 10: Integrated Case Study Simulation
Week 11: Career Strategy & Professional Branding
Week 12: Capstone & Networking Showcase
Admissions and prerequisites
-
Healthcare or life sciences background preferred
-
Comfort with MS Office and professional email etiquette
-
Laptop or desktop and reliable internet
-
Commitment to weekly classes and project work
Assessment and feedback
-
Rubric‑based marking with clear criteria
-
1 to 1 feedback on major submissions
-
One resubmission opportunity per assignment if needed
-
Completion requires all core deliverables submitted
Outcomes you can expect
Clarity on industry pathways and day‑to‑day expectations
Confidence working across Clinical, RA, PV and MA interfaces
Portfolio artefacts that show practical capability
Interview readiness and a personalised job strategy
Alumni network and ongoing support
Roles & indicative UK compensation
- Associate roles across Clinical, Regulatory, PV or Medical Affairs: £32k–£55k
- Contractor day rates vary by function and scope
Ranges vary by company, location and experience. No job guarantee.
“I finally understood how the pieces fit together.”
“Evening classes kept me consistent while working.”
Download the full syllabus
Get a detailed breakdown, sample assignments and rubric highlights
Prefer to speak to a human?
Book a 10‑minute discovery call to check fit, timelines and your goals.
Apply now
Why PharmaLink is different
- Evening schedule designed for working professionals
- Sixteen‑week completion buffer for quality work
- Simulated industry documents and realistic QC culture
- Direct coaching on rates, interviews and strategy
Refunds & deferrals
Simple policy summary here. Link to full policy page. Deferrals subject to availability.
Accessibility
We aim to support all learners. Tell us what you need and we will work with you.
No job guarantee
We provide training, coaching and references. Hiring decisions are made by employers.
Frequently asked questions
Who is this for?
When are classes?
Do I need prior pharma experience?
What support do I get?
Are there payment plans?
Apply now
Complete the short form and our team will follow up with next steps.
